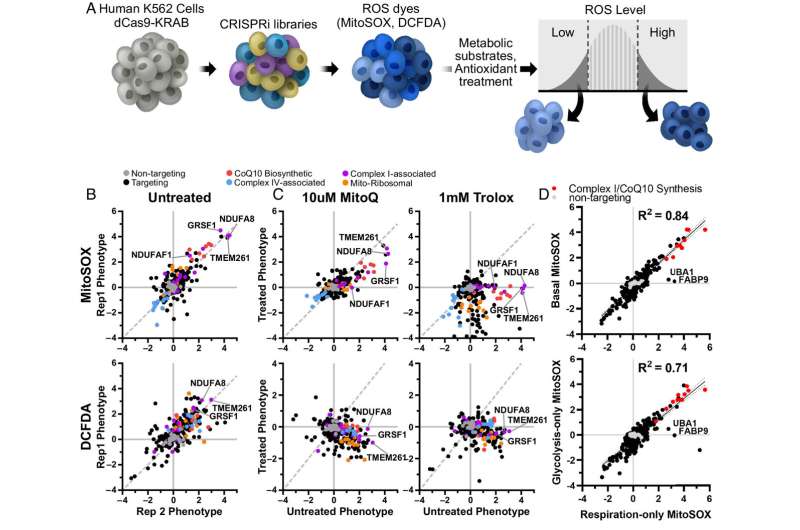
Is it possible to amp up the energy production of mitochondria without also boosting potentially harmful byproducts? If so, such a method could be used to treat a host of neurodegenerative diseases in which impaired mitochondria are believed to play a central role.
In pursuit of the answer, a team of scientists at Gladstone Institutes used the gene-editing technology CRISPR to parse out exactly which molecules are responsible for creating energy versus those that control the production of reactive oxygen species, or ROS—toxic byproducts commonly known as “free radicals.”
Their findings, which appear in Proceedings of the National Academy of Sciences, could lead to strategies for decoupling energy from ROS production, which may help in the development of therapies for diseases such as Parkinson’s or Alzheimer’s.
“Figuring out how to separate energy production from ROS production is really critical to treating mitochondrial dysfunction,” says Gladstone Investigator Ken Nakamura, MD, Ph.D., who led the study. “There are many conditions, including neurodegeneration, in which boosting mitochondrial energy could be beneficial, but we don’t want to damage cells through toxic byproducts.”
When mitochondria generate cellular energy from sugars and fats, they release ROS. Like pollution spewing from a power plant, ROS have long been considered unwanted but hard-to-prevent byproducts.
Though ROS serves some important biological functions, having too much of them around is toxic to cells and linked with many chronic and degenerative diseases.
Imbalance at the root of disease
Solving the question of how to help mitochondria operate more efficiently could contribute to new treatment approaches for neurodegeneration and conditions like heart disease, diabetes, and cancer. It even has implications for healthy aging, as mitochondria become faulty as we grow older.
However, in many cases, it’s tricky to figure out exactly how the mitochondria are malfunctioning: are they not making enough cellular energy, or are they making too much ROS?
Nakamura’s group previously screened cells to discover all the genes involved in regulating energy levels. In their new work, they focused on approximately 200 of those genes. Using CRISPR, they worked in cancer cells to selectively turn down the expression of each of those genes and studied what happened to ROS levels.
“We wanted to determine which molecules are required for energy production or ROS production,” says staff scientist Neal Bennett, the first author of the new PNAS study. “By doing that, we were able to discern the genes and pathways that can change those systems independently, which could be very helpful in treating disease.”
Indeed, although some genes affected both energy and ROS production, others had a much stronger effect on one product than the other.
Overall, these findings offer a compelling starting place for researchers who want to develop drugs that independently control mitochondrial energy and ROS and for those trying to understand how mitochondrial dysfunction is implicated in disease.
The team plans to carry out more research on the impact of altered ROS levels on cellular health and to determine whether their results hold true in other cell types, including brain cells.
More information:
Neal K. Bennett et al, Systems-level analyses dissociate genetic regulators of reactive oxygen species and energy production, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2307904121
Citation:
Cellular clean energy: Can mitochondria make more energy without collateral damage? (2024, January 12)
retrieved 12 January 2024
from https://phys.org/news/2024-01-cellular-energy-mitochondria-collateral.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

